NGS-Based Assays for Innovation in CGT Product Development
February 14, 2024
Marie-Ange Kouassi
The introduction of Next Generation Sequencing (NGS) technologies has transformed the field of Cell and Gene Therapy (CGT) research and development. As more therapies are being developed to treat specific conditions, it is important to have a reliable quality control procedure in place to ensure the safety of these products and to quickly provide solutions to patients. Due to the complexity of this product modality, a robust quality control process is necessary to ensure the safety of patients receiving these treatments.
With the cell and gene therapy (CGT) industry expanding rapidly, novel promising options are now available to treat previously incurable conditions. Patients suffering from life-threatening diseases can now have new hope and quality of life improvements. However, there are still several challenges hindering global access to these therapies such as the lack of identified critical quality attributes. For these therapies to achieve the desired purpose, ensuring their quality, stability, consistency, and comparability is crucial.1 Failure to maintain these attributes leads to low quality and unsafe treatment that could present health risks to patients and have costly consequences, in terms of litigation and damage to a company’s reputation. By leveraging advanced tools that enable efficient and accurate quality assessment, biopharmaceutical companies can mitigate risk. This will accelerate the delivery of life-changing treatments to patients who urgently need them.
Ensuring the Biosafety of Cell Therapies
During cell therapy development, there are many more points of entry for contamination with adventitious agents compared to gene therapy development. As these mammalian cells are cultured in animal-based media components or media containing animal-derived elements, there is a higher risk of infection.
It is therefore important to assess the biosafety of these cell therapy products at the earliest stages of the development processes - checking for contamination of:
- the starting material extracted from patients (autologous) or healthy donors (allogeneic), and
- any components e.g., media and cytokines, used to encourage their differentiation into specific phenotypes.2
Due to the invasive nature of the apheresis collections, only a minimum required amount of material is extracted for the therapy to limit patients’ physical and psychological stress. Therefore, the amount of material available for biosafety assessment is limited. Efficient biosafety testing approaches are required that provide valuable information while minimizing waste and preventing delays in product development.
Ensuring the Biosafety of Gene Therapies
Assessing the biosafety of viral vectors is of prime importance for gene therapies. Particularly before batch analysis, the likelihood of any cross-contamination should be evaluated. Additional data is also required by regulatory authorities such as the evaluation of contamination, the control, and stability during the development and characterization of cells used in the amplification of the genetic material.3 Also, a demonstration of the copy number and transfection efficiency is required to obtain regulatory approval for these therapeutic products.3 During gene therapy development, contamination may originate from the media as it contains animal-derived material. Production cells are another entry point of viral contamination and pose a high safety risk to patients if these viruses are pathogenic in humans.4 The CAACB (Consortium on Adventitious Agent Contamination in Biomanufacturing) of MIT’s Center for Biomedical Innovation collected a comprehensive dataset on viral contamination experience and reported contamination at all stages of the biological product life cycle.4 Such contamination can lead to discarded batches, interruptions in manufacturing, or may have legal implications, and cause delays in treatment administration to patients, potentially leading to death.
Genetic Characterization of CGTs for Identity and Stability Testing
During the development of such therapies, change is inevitable – e.g., changes in conditions, materials, and methods used. As living drugs that are sensitive and responsive to their environment, these changes (e.g., mutations) may affect their identity and stability creating difficulties in identifying and maintaining critical quality attributes. It is therefore recommended by the FDA to undergo identity and stability testing twice during the development process. Regular testing using characterization approaches that deliver rapid results (e.g., NGS), could be highly beneficial in monitoring product integrity for superior quality and efficacy. Through genetic characterization of CGTs, a wealth of information can be obtained to optimize stability and establish appropriate shelf lives and storage conditions for these products.
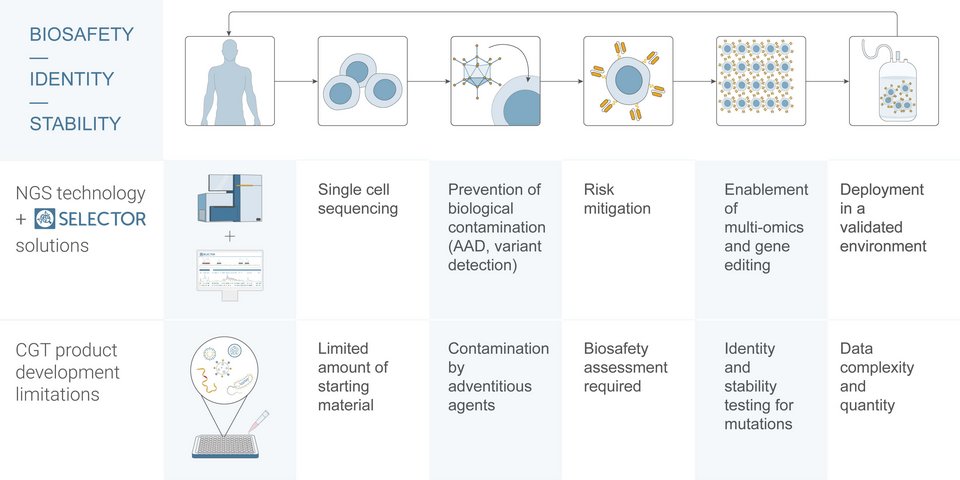
The Benefits of Choosing NGS for Quality Control
Leveraging current innovative and sensitive approaches enables biopharma companies to establish robust, optimized quality control processes during cell and gene therapy product development. This results in gains in time, reductions in costs, and improved patient safety. Incorporating NGS into the development process of CGTs presents several advantageous possibilities and is recommended by regulatory authorities.5 Unlike traditional gold standard methods, NGS-based biosafety assays allow rapid, accurate, and high-throughput detection of contamination by adventitious agents such as bacteria, mycoplasma, and viruses.1 This technique has a wider breadth of detection than other existing methods enabling targeted and untargeted adventitious agent detection (AAD).6 Besides biosafety, NGS provides a wealth of valuable information on CGT products through transcriptome analysis allowing thorough characterization of single cells or “living drugs”.7 However, implementing NGS at the earliest stage of the CGT development process is not easy, especially if companies do not already have this up and running in-house. It can be challenging to know how to design an NGS-based biosafety assay and establish optimized bioinformatics pipelines to analyze the data accurately for efficient decision-making.
Genedata Selector® for Streamlined NGS-Based Biosafety Workflows
To gain results rapidly at a reduced cost, biopharma companies often choose to outsource AAD assays for quality control to external service providers who offer PCR-based tests. This outsourcing requires sharing proprietary genomic information which can lead to risks associated with intellectual property (IP). Also, this technique limits the number of possible viruses that can be detected. NGS-based assays enable comprehensive biosafety assessment without compromising speed. Adopting Genedata Selector to implement NGS-based assays for biosafety in-house simplifies NGS-data analysis by providing optimized workflows and centralizing all genetic characterization for seamless collaboration and a single chain of custody. Designed specifically for biosafety and cell line development, the software has applications that are beneficial for both cell and gene therapy development.
Besides enabling accurate risk mitigation of contamination with adventitious agents from starting material to the final product, Genedata Selector also facilitates:
- omics or RNA-Seq-based quality control of cell therapies,
- machine-learning applications to predict characteristics of cells at different phases of growth during expansion and assess variability, and
- gene editing to enable verification of CRISPR results and gene insertion sites as well as the assessment of plasmid genetic stability.
Built for use in cGMP environments, Genedata Selector contains all information regarding samples for easy and transparent reporting and can also provide Computer System Validation (CSV) support allowing biopharma companies to eliminate bottlenecks and deliver life-changing therapies faster to patients.
Learn how you can accelerate cell and gene therapy product development with Genedata’s scientific experts and Genedata Selector.
Learn More about Genedata Selector
Authors:
Marie-Ange Kouassi, Ph.D., Scientific Communication Specialist, Genedata Selector
References:
- Stroncek, D. F., et al. Quality assessment of cellular therapies: the emerging role of molecular assays. The Korean Journal of Hematology. 45(1), 14–22 (2010).
- Dominic Clarke and Marie Aragon. Optimizing the quality of cell therapy starting materials. RegMedNet. (2018).
- EMA (European Medicines Agency). Guidelines on the quality, non-clinical and clinical aspects of gene therapy medicinal products. (2018).
- Barone, P. W., et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nature Biotechnology. 38(5), 563–572 (2020).
- WHO (World Health Organization). Proposed 1st International Virus Reference Standards for Adventitious Virus Detection in Biological Products by Next-Generation Sequencing (NGS) Technologies (CBER-5). (2020)
- Charlebois, R.L., et al. Sensitivity and breadth of detection of high-throughput sequencing for adventitious virus detection. npj Vaccines 5, 61 (2020).
- Tzani, I., et al. Tracing production instability in a clonally derived CHO cell line using single-cell transcriptomics. Biotechnology and Bioengineering. 118(5), 2016–2030 (2021).