Accelerating Vaccine R&D. Right Now.
Increase the efficiency and quality of vaccine research, development, and manufacturing.
The Genedata Biopharma Platform has been designed to increase the efficiency and quality of vaccine research, development, and manufacturing.
In addition to classical vaccine R&D approaches, it supports novel vaccine technologies such as synthetic self-amplifying mRNA, which accelerates vaccine development by utilizing synthetic and cell-free processes. This paves the way for the development of novel vaccines, even for antigens that are extremely difficult to produce and formulate. A dedicated, purpose-built enterprise software platform, it automates complex processes, supports teamwork in a division of labor environment, and integrates new R&D technologies and workflows. In particular, it optimizes vaccine bioprocess development, monitors vaccine product quality, and ensures data integrity and compliance
Enabling Next-Generation Vaccine R&D through Digitalization
The Genedata Biopharma Platform is a dedicated, purpose-built enterprise software system that accelerates vaccine discovery and development, automates complex R&D processes, supports teamwork in a division of labor environment, and integrates new R&D technologies and workflows. In particular, it supports:
- Novel protein- and peptide-based subunit vaccine design
- Innovative DNA and RNA vaccine engineering
(e.g., self-amplifying messenger RNA) - Novel adjuvants discovery
(e.g., via high throughput screening of immuno-potentiators) - Vaccine bioprocess development (including USP and DSP), formulation
- High throughput R&D processes
(e.g., systematic use of scale-down bioreactors) - Automation and robotics integration
(e.g., high throughput formulation screening) - Novel technologies
(e.g., polysaccharide vaccines, GMMA)
- Presentation and delivery
(e.g., alpha-virus vectors, vaccinia or baculovirus vectors) - Innate immune trigger identification
- Biosafety assessment
(e.g., NGS-based screens for adventitious agents) - Support of synthetic and cell-free processes
- Reverse vaccinology based on whole-genome analysis of the pathogen
- Bioconjugates management and testing
- Cancer vaccine discovery
Vaccine Discovery
Increase efficiency of vaccine research
- Manage the entire E2E workflow, from molecular biology to screening and production
- Produce and characterize recombinant nucleic acids, peptides, proteins and viruses
- Investigate novel antigens and identify relevant epitopes
- Streamline gene synthesis workflows for antigens and immunogens
Establish high-throughput discovery processes
- Automate and scale up cloning, screening, engineering, expression, and purification
- Integrate all lab instruments to automate data transfer, e.g., for screening and analytics
- Generate vectors in large-scale through bulk in silico cloning
- Ensure continuous process monitoring
Take an integrative systems biology approach
- Enable a holistic analysis of all data across projects and programs
- Facilitate communication between different teams and functions
- Optimize processes by systematically identifying R&D process bottlenecks
- Integrate new technologies via open architecture
Vaccine Development
Optimize vaccine bioprocess development
- Optimize yields and purities in expression and purification processes
- Integrate cell line selection, USP, DSP, formulation, and analytics workflows
- Streamline communication and handovers between different teams
- Automate all processes by directly integrating bioreactors or analytics devices
Monitor vaccine product quality and integrity
- Integrate all characterization and analytics data (e.g., antigens, protein sub-units, viruses, nucleic acids)
- Identify potential developability risks based on integrated product quality assessment
- Correlate manufacturing process parameters with product quality; full QbD support
- Generate full historical record for each vaccine candidate in a single mouse click
Ensure data integrity & compliance
- Enable full traceability from raw materials to final product using comprehensive sample barcoding
- Establish a single-source-of-truth by providing real-time access to all project information
- Rely on an established enterprise platform based on industry standard architecture
- Enforce data integrity and compliance due to centralized data management
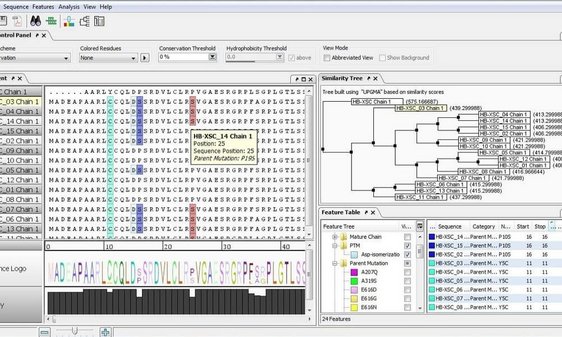
Streamlining vaccine research: Example of a systematic sequence comparison of different subunit proteins HepB-XSC. All sequences are automatically quality controlled, translated, analyzed, and annotated on both the DNA as well as the protein level, and critical mutations are highlighted.
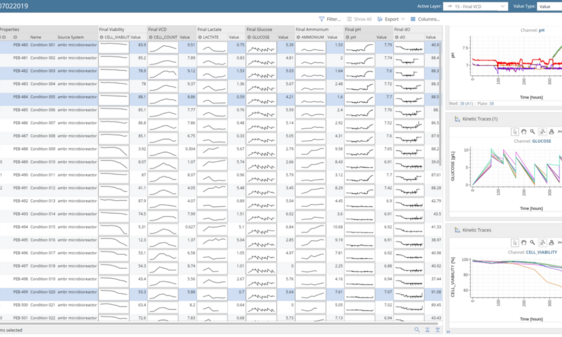
Accelerating vaccine bioprocess development: Systematic analysis of different online and offline parameters in bioreactor runs showing time profiles of various critical parameters (e.g., titers, pH, feed) that are automatically captured and processed. The system supports all bioreactor scales including downscaling platforms (e.g., Sartorius’ ambr, Eppendorf’s DASGIP, Pall’s Allegro bioreactors).