強力なデータインベントリで異なる研究開発チームを組織横断的につなぎ、トランスレーショナルリサーチを促進
June 9, 2022
Marie-Ange Kouassi
With evolving drug modalities, newly emerging technologies, and critical health needs, the amount and complexity of data generated during drug development is rapidly increasing. But without its conversion into easily accessible knowledge, its impact is limited. To accelerate precision medicine to improve human health, having all the right data at the fingertips is crucial. Genedata Profiler offers a powerful data inventory that facilitates this.
Deriving actionable scientific insights that translate into life-changing therapies depends on having an integrated view of all relevant datasets and data types. This can be achieved using a catalog system to easily discover and access data no matter which source it originates from. Such a system is known as a data inventory and contains various types of data objects along with metadata describing their content and any other useful information. It facilitates effective collaboration and seamless data distribution throughout an organization between different team members, different teams, and even external partners in a way that maximizes the value of data and allows the right decisions to be made. Particularly for the development of precision medicines, biopharmaceutical companies need to apply knowledge from previous studies to better understand patient-specific differences and identify the right therapy for the right patient. With a translational research data inventory, Genedata Profiler® supports the design of innovative clinical trials and the development of effective precision therapies by eliminating virtual barriers and harmonizing multi-dimensional data to enable the discovery of novel insights through advanced analytics.
Why Use Data from Different Sources?
There is power in gathering, integrating, and analyzing the diverse data types (including multi-omic, phenotypic, clinical & imaging data) emerging from preclinical, clinical, and real-world evidence (RWE) studies. Collectively, when analyzed, they allow biopharmaceutical companies to answer important questions such as:
- Which patient will benefit from which treatment?
- Does this disease exist in different subtypes? How do they differ in their mechanism?
- How will this patient be affected in the long term?
These insights spur the development of more targeted therapies and the discovery of new indications for existing ones- the foundation of drug repurposing. To accelerate the translation of research into clinically approved products beneficial for human health, governmental bodies worldwide are also encouraging the sharing of data between institutions. For biopharmaceutical companies, this means not only managing the vast amounts of complex data generated internally from target identification to clinical trials but also licensed and publicly available data from other institutes, biobanks, and more. This presents a host of challenges when it comes to their management as the data needs to be stored in a way that allows continuous fruitful use yet maximum security.
Why Is Managing Data from Different Sources Challenging?
The various data used during drug development are generated by geographically dispersed parties and therefore stored in disparate locations making it challenging for collaborators to re-use. Not only are these data diverse in format and structure but they can have unique nomenclature and inconsistent/missing metadata labels making them difficult to find, combine, and compare. There are also a limited number of data management and analytics tools available that enable different types of data consumers to easily explore the combined data for their downstream needs.
As some of the data should not be available to all the different parties involved in a project, this data should be restricted, however, if this data is prepared and controlled by data stewards in a way that complies with regulations, it could be highly valuable for various data consumers. Achieving this fine-grained access and data handling activity control through data ownership while facilitating easy data access is not an easy task.
These challenges hinder R&D productivity and the ability of biopharma companies to innovate potentially diminishing their overall return on investment. A data culture that marries data literacy, “the ability to draw valid conclusions from data including understanding the limits and awareness of common biases”, and data governance, “the process by which datasets are managed to ensure trustworthiness and compliance with regulations”, is required. Essentially, the data gathered by different biopharmaceutical organizations needs to be easily discoverable for a specific purpose. To be able to apply these data, it needs to be well connected to analytical tools allowing for performant visualization to answer specific questions on time.
Genedata Profiler, a Translational Research Data Inventory for Precision Medicine
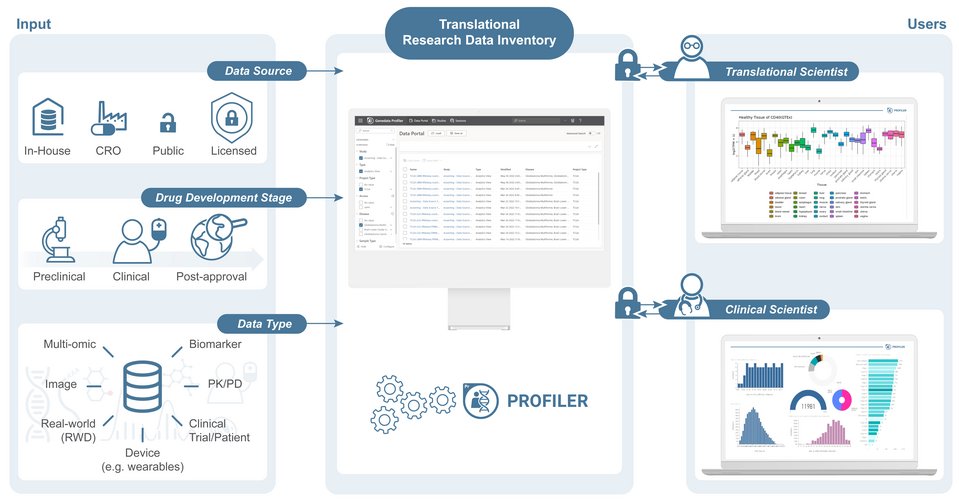
Genedata Profiler is a Bio-IT Innovative Practices Award-Winning data integration and analytics platform that enables the FAIR principles. The validation-ready software is based upon a performant analytics architecture that allows molecular, biomarker, omics, imaging, phenotypic and clinical data to be easily found and accessed for further analysis. Built in a user-centric manner, the software enables the sharing of fit-for-purpose data between various data consumers including but not limited to Translational Research Scientists and Clinical Scientists while governing data access.
These key roles explore different research questions by navigating between studies from various stages of drug development. Also crucial to their work is being able to compare findings from data generated in-house with other publicly available or commercially sourced data, validate hypotheses, and generate new hypotheses.
With Genedata Profiler’s powerful data portal which functions as a data catalog, translational research scientists and clinical scientists can easily find the right dataset using an “amazon-like” search based on filters. These pre-set filters by data stewards allow any user to retrieve the data they need for their specific purpose given they have authorized access. With Genedata Profiler, translational research scientists and clinical scientists also benefit from investigating data ingested by their organization’s data stewards such as TCGA, CCLE, GTEx, and commercially available data such as TEMPUS and FlatIron Health. Thanks to the automatic labeling with metadata enabled by Genedata Profiler which allows for superior data organization, these data consumers can easily and efficiently discover the data they require.
Once the data of interest has been found, these individuals can directly load the data in their desired analytics tools for cross-study analysis, or they can prepare and publish these datasets to team members/partners. The seamless connection to application-specific dashboards allows for easy visualization of updates to data and the generation of insights for accelerated decision-making. Genedata Profiler consists of inbuilt expert tools for advanced statistics and artificial intelligence yet has a growing repertoire of integrated analysis and visualization tools from RStudio and Spotfire to PowerBI.
Accelerating Innovation for Precision Medicine
A powerful data inventory for translational research has clear advantages for a biopharmaceutical company developing precision medicine. As a field that requires heavy inter-and intra-disciplinary collaboration, as well as the application of data-driven insights from different phases of drug development and other organizations, centralizing and organizing all data for effective and efficient re-use is essential.
Genedata Profiler democratizes data access across the Translational & Clinical research ecosystem connecting teams and external partners for unified collaboration. The software provides ownership to ensure data is protected and its sharing is controlled for handling only by authorized team members. In the platform, all gathered data is queryable from the data inventory in a scalable manner for seamless conversion into scientific insights that inform decision-making. This way, biopharmaceutical companies increase R&D productivity and unlock insights that guide the development of effective precision therapies against disease.
Contact us to implement a translational research data inventory in your organization.
Author: Marie-Ange Kouassi, Ph.D., Product Marketing Specialist, Genedata Profiler