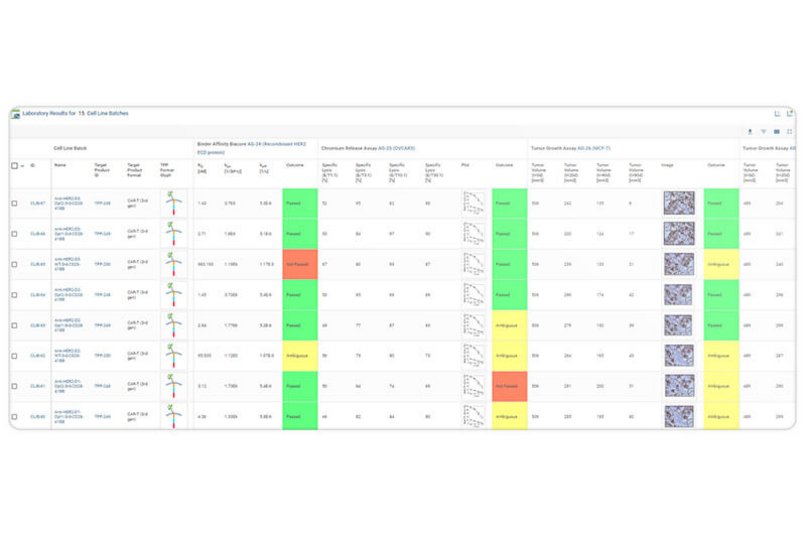
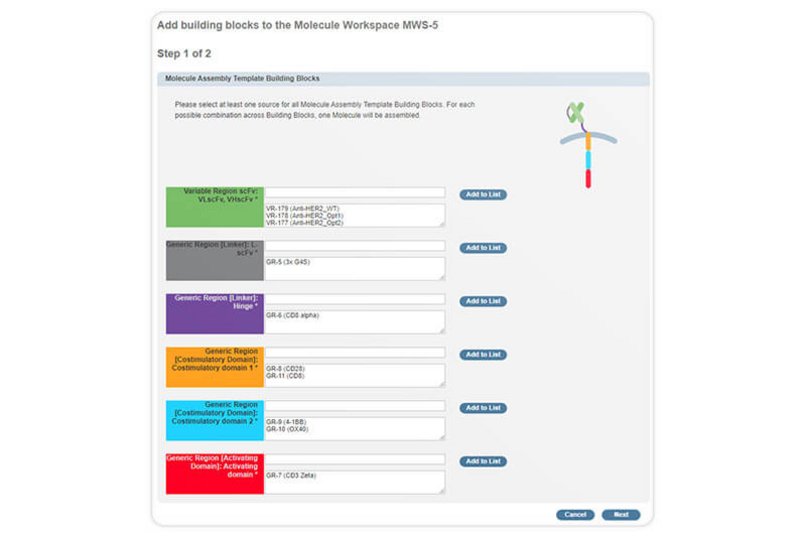
With purpose-built functionalities for the design and evaluation of large panels of cell-based therapeutic modalities for novel immunotherapies, such as chimeric antigen receptors T cells (CAR-T), T Cell Receptor Technology (TCRs), and antibody TCR-mimetics, Genedata Biologics® enables the systematic engineering, production, and testing of novel therapeutic modalities.
CAR-Ts. TCR-Ts. NKs. TILs.
Genedata Biologics streamlines the full process from design, generation, testing, and validation, to developability and manufacturability assessments. By directly integrating with laboratory equipment, it fully automates sample handling and testing procedures. An integrated database and tools support screening and engineering of a variety of TCR-based formats (e.g., dsTCR, TCR-scFv, bispecific TCRs).
Customer Stories

Building on Genedata for Cell Therapy Innovation
"The implementation of the Genedata platform has helped us to digitalize Immatics’ TCR-related R&D. This means that time-consuming paperwork is minimized, and we can easily find information when it is needed."

Streamlining Cell Therapy Research and Development
"Given their track-record of success, Genedata’s platform was the clear choice to support and integrate our full cell therapy discovery and development efforts.”

Accelerating Development of TCRs and Bispecifics
“Genedata Biologics is a perfect fit for our approach, because… we anticipate that the platform will be able to handle our bispecific engineering and TCR discovery and optimization workflows without any customization.”