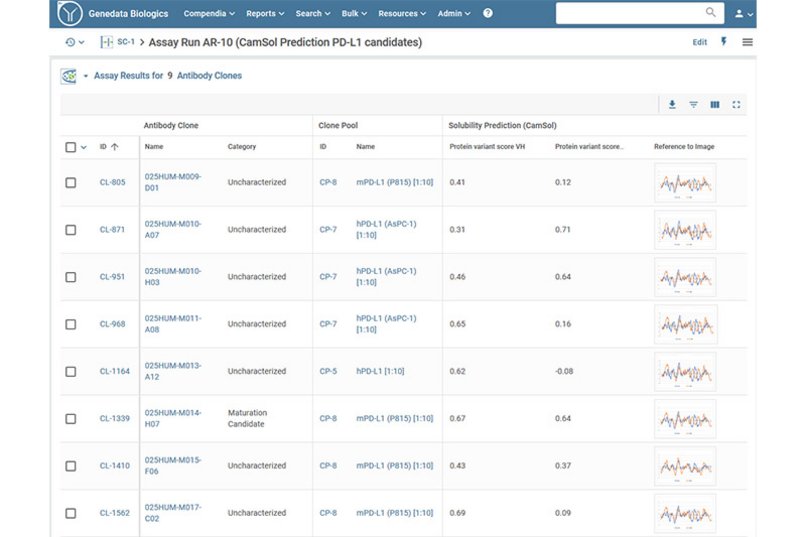
Genedata Biologics® enables the systematic assessment of a candidate’s developability risk profile and critical quality attributes (CQA) to decide on the most promising development candidates. Common problems identified include aggregation issues, formulation instability, and reduced pharmacological activity.

Developability & Manufacturability
Genedata Biologics assesses a drug’s manufacturability in terms of potential problems such as those during expression and purification. The platform also evaluates properties that affect molecule robustness on a biophysical and biochemical level. Typical tests include stability (e.g., pH, freeze-thaw, high- and low-temperature stability tests), degradation (e.g., resulting from extreme pH values, light, oxidation), viscosity, thermal stability, solubility, glycosylation characterization, and immunogenicity. The platform evaluates intrinsic molecular properties that may impact technical development and comes with built-in tools to enable a systematic and holistic assessment of all drug candidates to minimize future development and manufacturing risks.
Customer Stories

Streamlining Antibody Discovery Processes
“Genedata Biologics will be our central repository for mission-critical data on therapeutic candidates such as bioactivity, developability, and manufacturability, and will enable transparent decision-making on candidates to move forward with.”

Managing Developability & Manufacturability Risks
We present a scalable, off-the-self enterprise workflow system that enables systematic developability and manufacturability assessments from the very early stage to the later stages of the biopharma R&D process.